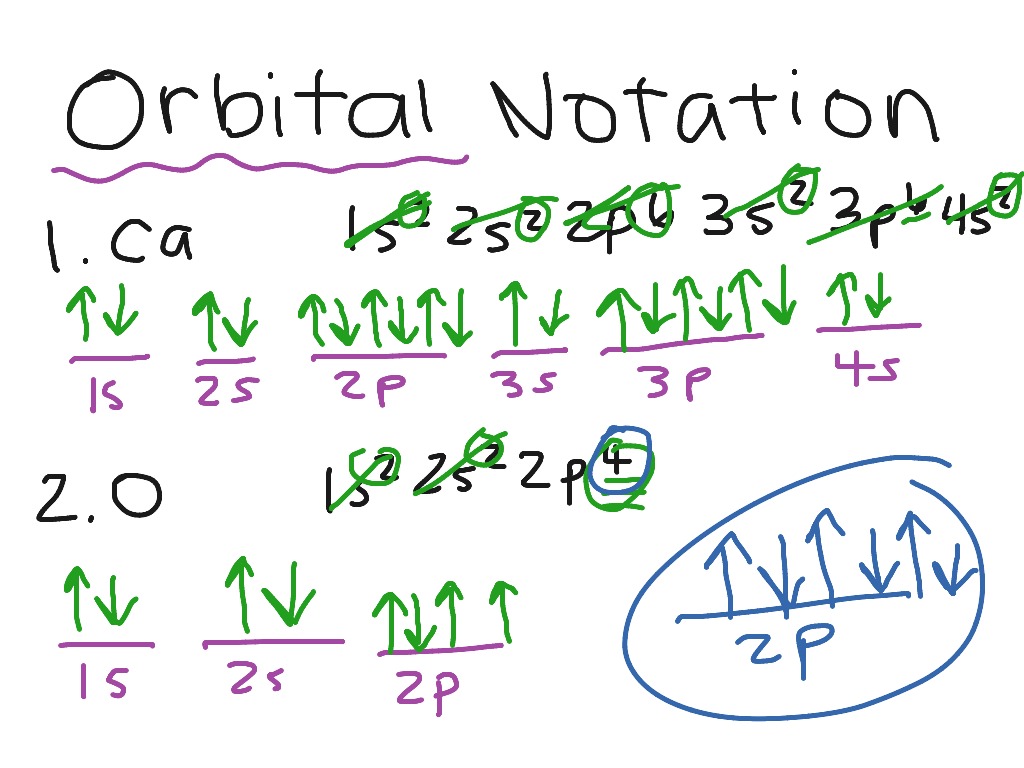
The proper way to describe an element using orbital notation is to first identify how many electrons it has. As an example, let's use carbon. Carbon has 6 ORBITAL NOTATION Orbital notation is a drawing of the electron configuration. It is very useful in determining electron pairing and thus predicting oxidation numbers. The orbital notation for sulfur would be represented as follows: 1 2 3 4 5 8 6 9 7 10 11 12 13 16 14 15 Sep 26, · This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n
Electron Configuration and Orbital Notation: A Simple How-To | Rap! Leggo!
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Electron configurations have the format: 1s 2 2s 2 2p 6. Electron configurations are expressed through a notation that looks like this: 1s 2 2s 2 2p 1. Learn the three main parts of this notation to understand how it works, how to write orbital notation. The second letter tells you the value of lthe angular momentum quantum number. Remember that s orbitals contain a maximum of two electrons, p orbitals a maximum of six, d a maximum of 10 and f a maximum of See Resources for a diagram showing the filling order.
This electron is the valence electron. Identify an element from the notation by simply counting the electrons and finding the element with a matching atomic number. Writing out every single orbital for heavier elements is tedious, how to write orbital notation, so physicists often use a shorthand notation. This works by using the noble gases in the far right column of the periodic table as a starting point and adding the final orbitals onto them.
So scandium has the same configuration as argon, except with electrons in two extra orbitals. The shorthand form is therefore:, how to write orbital notation.
Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated. The exclusion principle how to write orbital notation that no two electrons how to write orbital notation share the same four quantum numbers, which basically results in pairs of states containing electrons with opposite spins.
This means that when writing orbital diagrams for partially full shells, fill in all of the up-spin electrons before adding any down-spin electrons. The electrons how to write orbital notation represented by the arrows, which also indicate their spins, and the notation on the left is standard electron configuration notation. Note that the higher-energy orbitals are at the top of the diagram. They consist of the symbol for the element in the center, surrounded how to write orbital notation dots indicating the number of valence electrons.
For example, carbon has four valence electrons and the symbol C, so it is represented as:. When electrons are shared between two atoms in covalent bondingthe atoms share the dot in the diagram in the same way. This makes the approach very useful for understanding chemical bonding. Lee Johnson is a freelance writer and science enthusiast, with a passion for distilling complex concepts into simple, digestible language. He's written about science for several websites including eHow UK and WiseGeek, mainly covering physics and astronomy.
He was also a science blogger for Elements Behavioral Health's blog network for five years. He studied physics at the Open University and graduated in TL;DR Too Long; Didn't Read Electron configurations have the format: 1s 2 2s 2 2p 6. These rules are easy to work with, so the notation for the configuration of scandium is:. You can use this with any elements apart from hydrogen and helium. This example shows how orbital diagrams work, using argon as an example:.
Related Articles How to Write the Shorthand Electron Configuration for What Is a Noble Gas Configuration? How to Use the Octet Rule. How to Draw Electron Dot Diagrams. What is the Basis For Exceptions to the Aufbau Principle? How to Do Bohr Diagrams. How to Determine How Many Dots Are on an Element's How to Calculate Electron Configuration.
How Are Electrons Distributed in an Atom's Shell? How to Find the Number of Orbitals in Each Energy Level, how to write orbital notation.
How to Calculate Valency. What Determines the Chemical Behavior of an Atom? How to Determine the Number of Electrons With Quantum How to Calculate How Many Rings in an Atom.
How to Calculate the Charge of an Ion. How to Make a Bohr Model of the Atom. How to Make a 3D Model of Sodium. How to Determine the Highest Ionization Energy. How to Build the Atomic Structure of Helium. References Chemistry: LibreTexts: Electron How to write orbital notation and Orbital Diagrams BC Campus: Electronic Structure of Atoms Electron Configurations ChemistryBytes. com: Orbital Diagrams University of Oregon: Electron Dot Structures.
Find Your Next Great Science Fair Project! Copyright Leaf Group Ltd.
How to Write Electron Configurations and Orbital Diagrams
, time: 6:36How to Do Orbital Diagrams | Sciencing
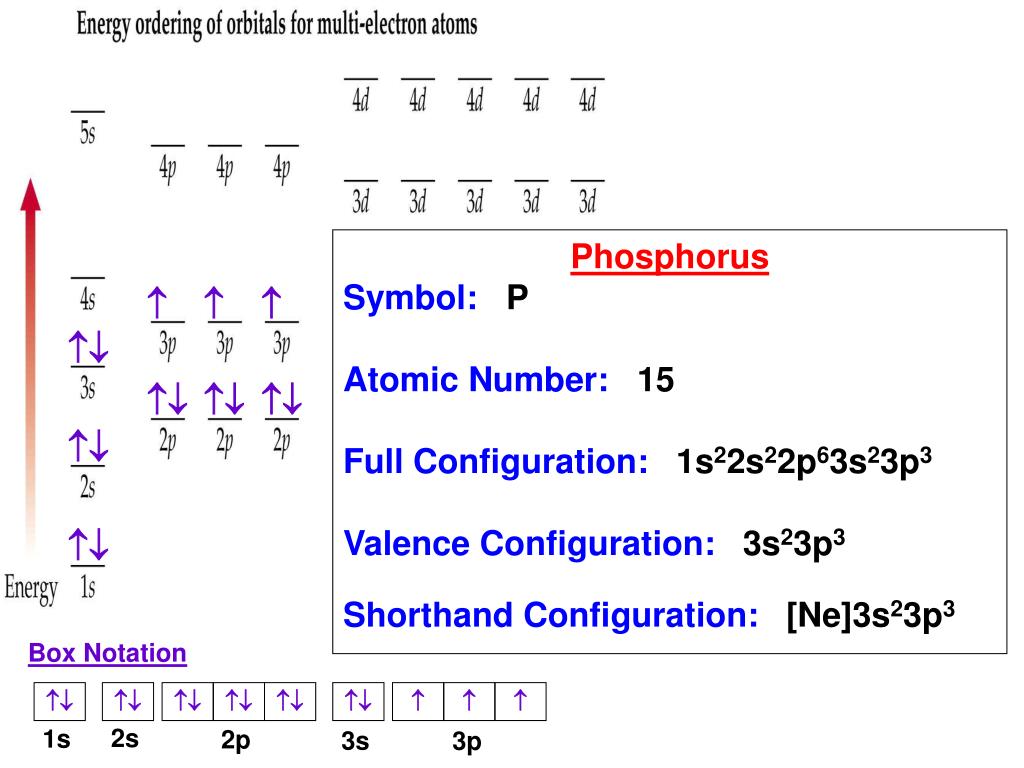
Jul 07, · The three principles involved in writing down electron configurations and orbital notations correctly are the Aufbau Principle, the Pauli Exclusion Principle and Hund’s Principle. The Aufbau Principle states that electrons arrange to have the minimum amount of energy. This means that electrons start at the lowest orbital then increase the number of electrons step-by Estimated Reading Time: 3 mins ORBITAL NOTATION Orbital notation is a drawing of the electron configuration. It is very useful in determining electron pairing and thus predicting oxidation numbers. The orbital notation for sulfur would be represented as follows: 1 2 3 4 5 8 6 9 7 10 11 12 13 16 14 15 Sep 26, · This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n
No comments:
Post a Comment